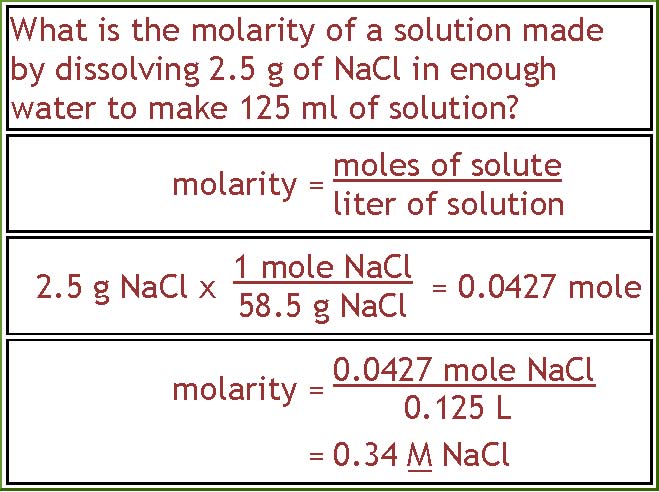
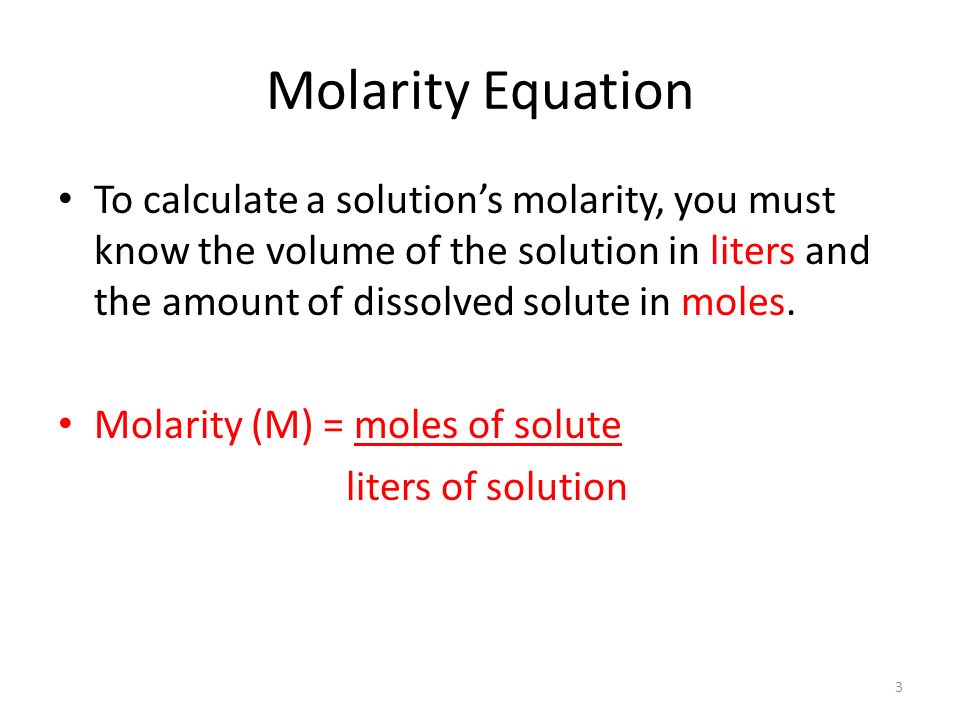
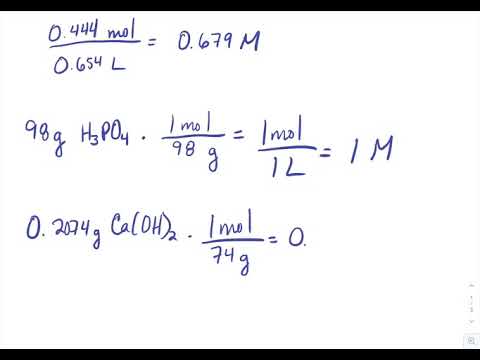
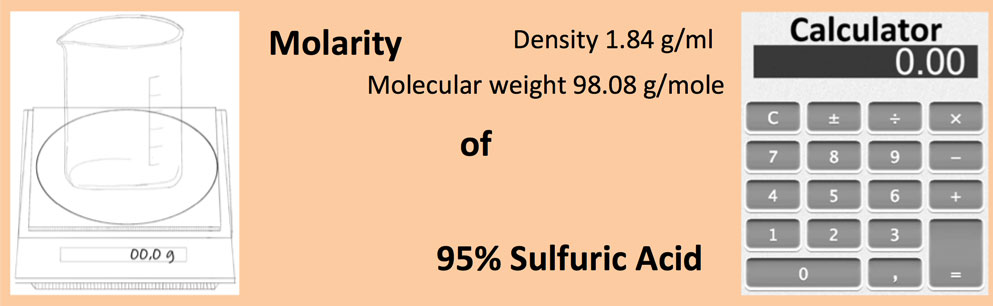
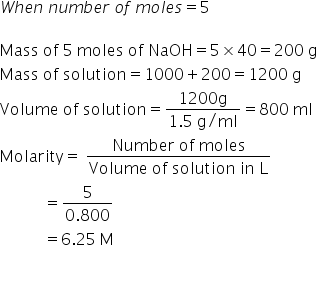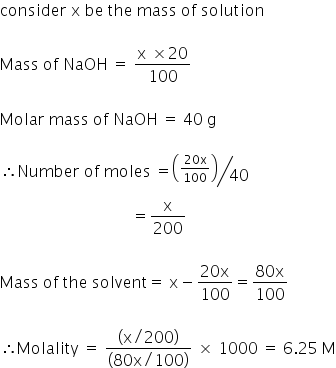A solution is prepared by dissolving 4g of NaOH to give 500 mL of it. Calculate the molarity of the solution.
A solution has 20% NaOH (w/w) and the density of the solution is 1.2 g/mol. What is the molarity of the solution? - Quora

Calculate molarity of NaOH in a solution made by mixing 2 L of `1.5 M NaOH,3 L` of 2 M NaOH and 1L - YouTube

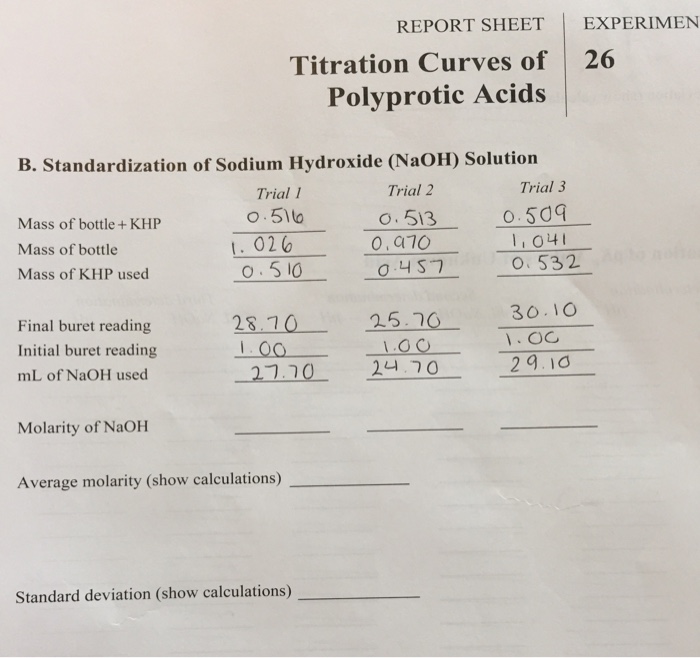
OneClass: Can you please help me make sure the Molarity of NaOH based onmy answers from the final-ini...
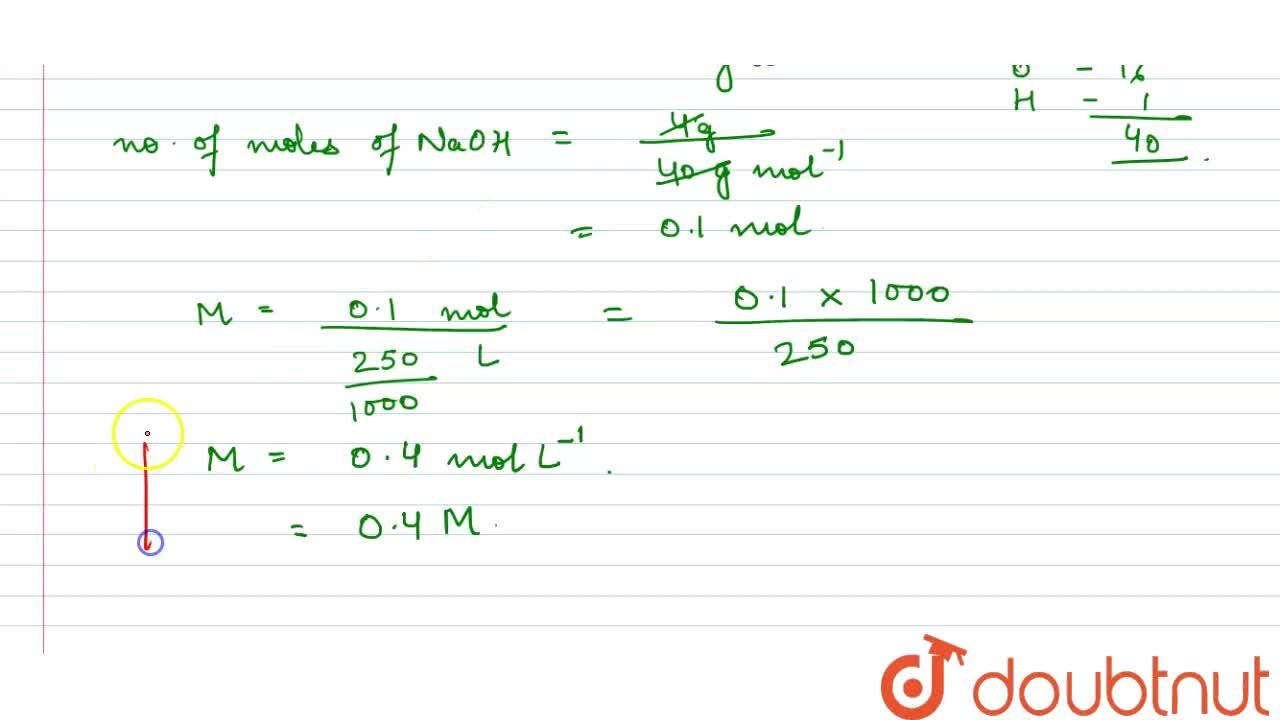
Calculate the molarity of NaOH in the solution prepared by dissolving its 4 g in enough water to form 250 mL of the solution.